Background:
Physical, chemical, biological, and geological marine and coastal research are crucial for understanding the world’s oceans. This research is ideally accomplished through continuous long-term monitoring to understand the characteristics and processes in the marine environment. The average salinity of the world’s oceans is 34-36 ppt with many oceanographic-based sensors being tailored to operate under these conditions.
However, there are regions in the world’s waters where high evaporation and low precipitation occurs resulting in an increase in salinity from 36 to 40 ppt. The Red Sea is one such prime example being one of the most saline water bodies in the world. The Red Sea is almost completely landlocked with water flow either from the narrow Suez Canal in the North or the Bab El-Mandab Strait in the South. Moreover, as precipitation is almost non-existent there, there is little inflow of fresh water, which is further compounded by heavy evaporation caused by the extreme heat in this region. The salinity of the Red Sea, therefore, ranges from ~36 ppt in the southern part and 41 ppt in the northern part.
The Mediterranean Sea is another of the most saline seas in the world, with a salinity of ~38 ppt in the west and up to 40 ppt in eastern regions in the summer. This is due to the rate of evaporation being three times higher than the influx of freshwater. The waters of the Mediterranean Sea have become saltier and warmer for the past 40 years at rates of about 0.015 ppt and 0.04 °C per decade (M. Borghini et al.), with the rate predicted to further increase in the next decades.
The Problem:
Measurement of pH is key to understanding marine chemistry and biology and is of significant interest in areas of these extremely high salinity conditions. However, despite it’s importance, today’s pH sensors are not fit for purpose for monitoring in these environments. In the case of potentiometric-based sensors such as ISFET or pH glass electrodes, the difference in ion concentration between the reference electrode chamber and the outside system will generate an unknown and uncalibrated liquid junction potential. Over prolonged use, ion exchange through the porous frit will occur altering the composition of the reference solution. This causes a shift in the reference potential impacting the calibration algorithms and the ultimate accuracy of the sensor.
An alternative to potentiometric technology is the colorimetric-based sensor, whereby seawater is mixed with pH indicator dyes and the spectrophotometric response is recorded. However, the algorithms used to determine the pH from the response are highly dependent on the salinity of the solutions, so in high concentrations of chloride or sulfate ions, incorrect pH measurements result.
ANB’s Solution:
ANB has conducted a number of laboratory experiments with their pH sensor in conditions of high salinity where today’s technologies are not suitable: synthetic seawater covering the range 25 to 41 ppt. The figure below shows the pH as a function of time and shows that the ANB sensor measures pH across that salinity range.
The accuracy of the measurement has been validated using a spectrophotometer (Jenway) using meta-Cresol Purple as an indicator dye. The data is presented in figure 1.
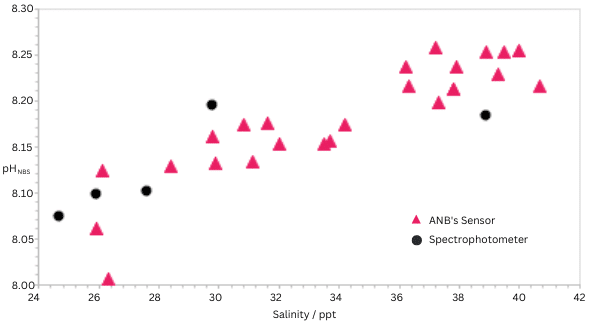
The data presented is entirely consistent with previous data by A.K. Covington et al. who showed the pH of a solution increases with salinity. ANB’s internal algorithms are calibrated using the National Bureau of Standard (NBS) pH scale. The sensor is calibrated against a freshly calibrated glass electrode. The spectrophotometer measurement provides the pH using the total hydrogen ion concentration scale. In the data in figure 1 the ‘total’ pH scale used for the colorimetric technique has been corrected to the NBS scale (S. Aßmann et al.) with excellent agreement shown between the techniques.
In order to confirm the efficacy of ANB’s sensor to perform in the full salinity range, the next step is to move from laboratory experiments to field tests. For that, and with the aid of Jerico funding, ANB is collaborating with the Hellenic Centre for Marine Research (HCMR) based in Greece, to study the performance of the sensor in the high-salinity and high-temperature waters found locally. The researchers at HCMR are experts in long-term marine and coastal monitoring and research, so watch this space for new data!