For millions of years the pH of the oceans has remained constant, allowing marine life to evolve and be tailored to the chemical balance. However, the oceans are estimated to have absorbed about 30 per cent of the emitted carbon dioxide from human activities since pre-industrial times. The carbon dioxide is contained in the upper 10 per cent of oceans (less than 1000 metres depth) because of slow ocean mixing processes. As CO2 is absorbed the concentration of hydrogen ions is increased and the pH decreased. A decline of 0.1 from pre-industrial times has already been recorded in the pH of the oceans, corresponding to a 26% increase in acidity. However, despite the evidence behind ocean acidification, the data are limited in both coverage and quality and there is a need to develop complete sensor suites to monitor the oceans carbonate cycle, pH being the key parameter.
What is Ocean pH?
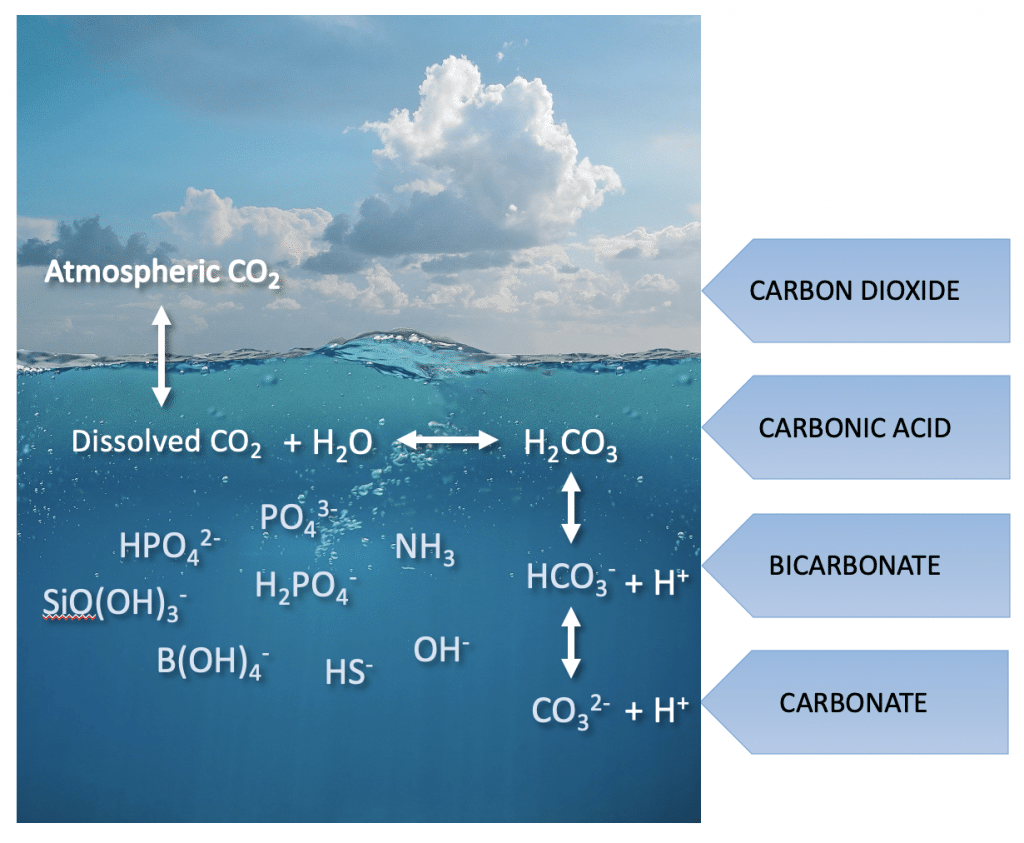
Ocean pH is set by the carbonate concentration in the ocean, which is in dynamic equilibrium with atmospheric CO2 concentration. Atmospheric CO2 is absorbed into the ocean and once dissolved, undergoes a rapid reaction with water to form carbonic acid. The equilibrium reactions below show how the pH is set in the ocean.
CO2(aq) + H2O ⇔ HCO3–(aq) + H+(aq)
HCO3–(aq) ⇔ CO32–(aq) + H+(aq)
At seawater, pH (8.2), the distribution of the three species, CO2(aq), HCO3–(aq), and CO32–(aq) is 0.5%, 89%, and 10.5%. This can be rationalized as follows, the first equilibrium is dominated by the concentration of bicarbonate in the solution, with very little CO2(aq) left unreacted in solution. In contrast the second equilibrium favours the reactant side, and as such the bicarbonate ion, HCO3–(aq) is the major species in the ocean.
This is exemplified by the fact that global ocean climate datasets like the World Ocean Circulation Experiment (WOCE), Hawaii Ocean Time-series (HOT), and Bermuda Atlantic Time-series (BATS) have only included carbon variables since the late 1980s. Typically large-scale sampling efforts and long-term time series have mostly concentrated on the open ocean. Not unsurprisingly coastal shallow water experience higher variation in pH and represent 10–20% of the oceanic CO2 sink.
Impact
Ocean acidification reduces the concentration of carbonate ions having an extreme impact on the Oceans Health. Carbonate ions are the building blocks for many marine animals such as corals, oysters, clams, sea urchins, molluscs, crustaceans and echinoderms, helping them to produce shells and skeletons, as the concentration of carbonate ions reduces, their health decreases. Indeed, reef development is thought to cease at pH 7.8. This environmental impact on foundational species like coral, phytoplankton, and shellfish will have cascading effects on community structure, food, biogeochemical cycling, and commercial fisheries.
Ocean acidification is forecast to cost the global economy around $1 trillion annually by 2100[1] through negative impacts on ecosystem services. Coral reefs provide a global economic value of $30 billion per year and the loss of them through ocean acidification is projected to cost tens of billions of dollars annually (or 0.18% of global GDP) by 2100.[2]
[2] Makarow et al., 2009